oaklodgefostering.ie
Oak Lodge Residential & High Support Fostering Service. Chapel Street, Carrick-on-Suir, Co. Tipperary, Ireland. (Please tick the type of placement required) Residential Care Name of H.S.E. Local Authority making referral: This referral has been formally approved for placement by the appropriate H.S.E. local authority and funding agreed. PLEASE ENSURE ALL SECTIONS ARE COMPLETED. Page 1
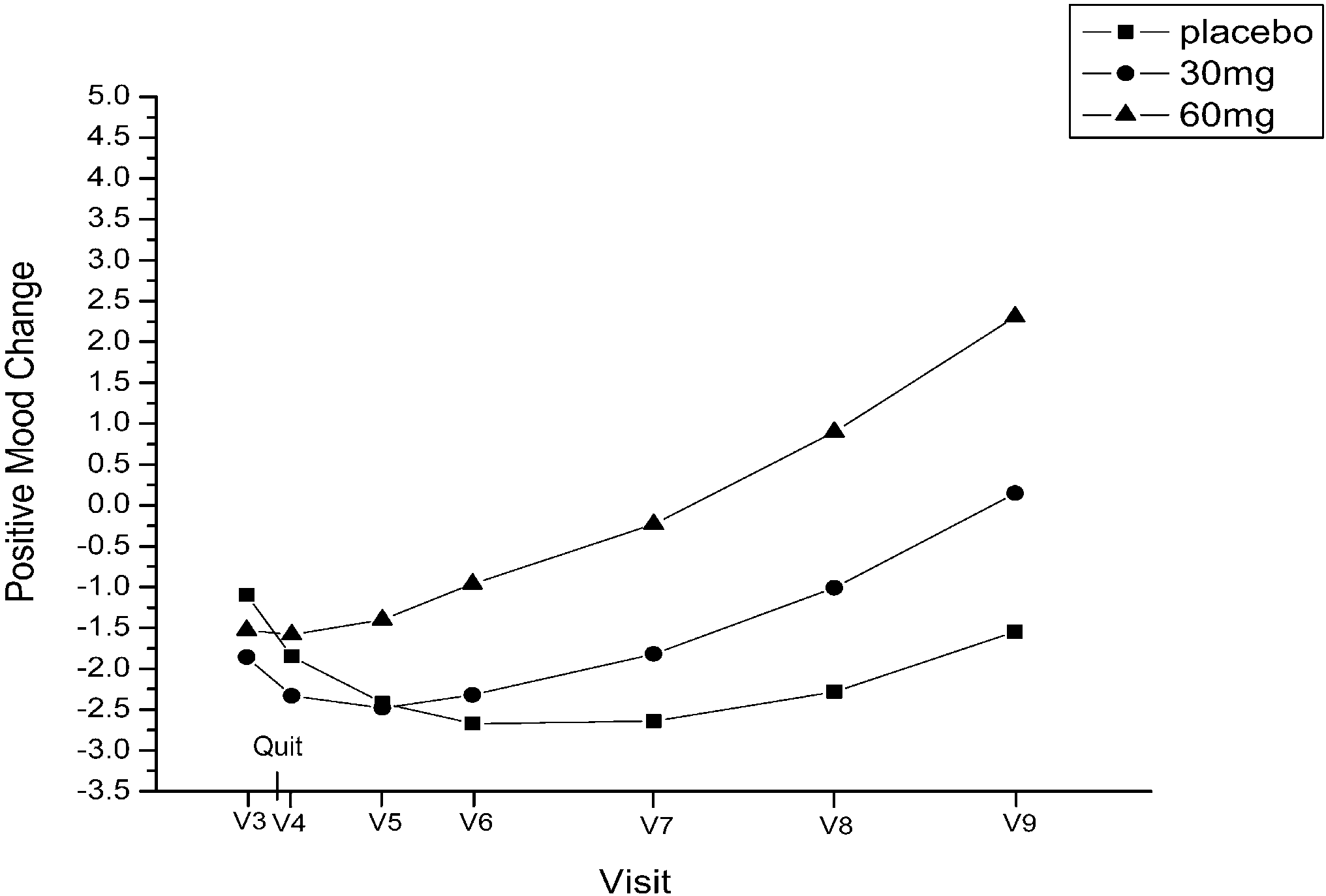 on 60 mg. At visit 9, abstinent smokers comprised 21.7%of the placebo group, 14% of the 30 mg group, and 24.9%of the 60 mg group.
on 60 mg. At visit 9, abstinent smokers comprised 21.7%of the placebo group, 14% of the 30 mg group, and 24.9%of the 60 mg group.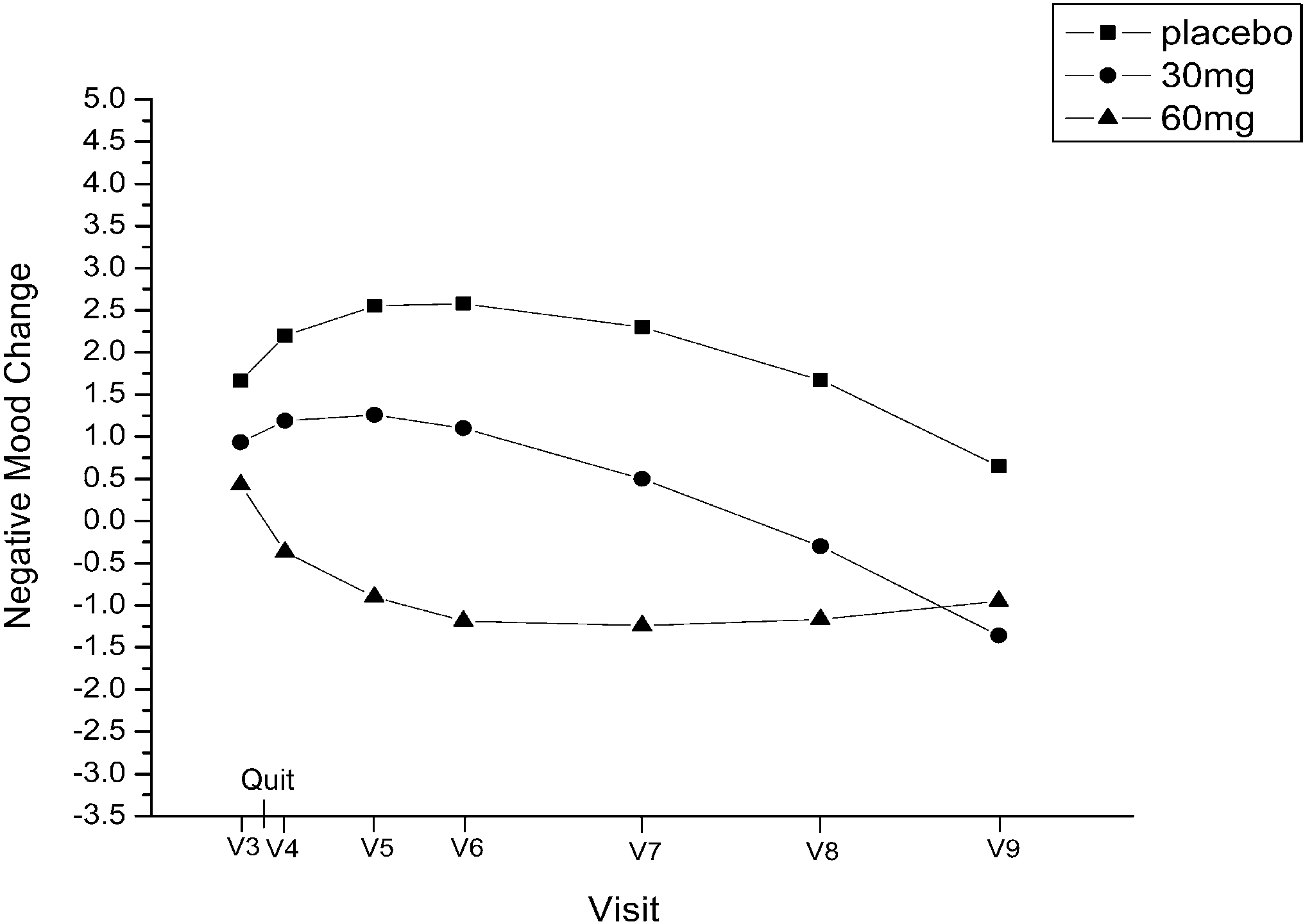 negative affect from visit 3through visit 9, determined by
negative affect (linear, P=0.04; quadratic, P=0.04). Re-sults suggest that positive and negative affect changes areinfluenced by fluoxetine rather than feelings of successafter quitting smoking.
negative affect from visit 3through visit 9, determined by
negative affect (linear, P=0.04; quadratic, P=0.04). Re-sults suggest that positive and negative affect changes areinfluenced by fluoxetine rather than feelings of successafter quitting smoking.