The official proceedings of the regular gering city council meeting, may 10, 2010
THE OFFICIAL PROCEEDINGS OF THE REGULAR GERING CITY COUNCIL MEETING, AUGUST 23, 2010 A regular meeting of the Gering City Council of Gering, Nebraska was held in open session at 7:00 p.m. on August 23, 2010. Present were Mayor Wiedeman and Councilmembers Smith, Christensen, Gibbs, McFarland, Hillman-Kortum, Shields, Morrison and Escamilla. Also present were City Administrator Danielzuk, Cit
 Androgen responsiveness of L T2 cells · M A LAWSON and others 603
Although direct activation of AR leads to transcriptional
responses dependent on the interaction of ligand-bound AR with a competent promoter element, othermechanisms for AR-dependent activation of transcriptionhave been reported that are resistant to inhibition by theandrogen receptor antagonists 2-hydroxy-flutamide andcasodex (Peterziel et al. 1999). Therefore, to establish thatthe androgen responsiveness of pGL3-MMTV is depen-dent on the transcription activation properties of ligand-bound AR, we tested the ability of the AR antagonists2-hydroxy-flutamide and casodex to block the DHT-
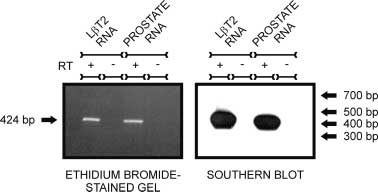
Figure 1 L T2 cells express AR mRNA. Primers specific for mouse
Androgen responsiveness of L T2 cells · M A LAWSON and others 603
Although direct activation of AR leads to transcriptional
responses dependent on the interaction of ligand-bound AR with a competent promoter element, othermechanisms for AR-dependent activation of transcriptionhave been reported that are resistant to inhibition by theandrogen receptor antagonists 2-hydroxy-flutamide andcasodex (Peterziel et al. 1999). Therefore, to establish thatthe androgen responsiveness of pGL3-MMTV is depen-dent on the transcription activation properties of ligand-bound AR, we tested the ability of the AR antagonists2-hydroxy-flutamide and casodex to block the DHT-
Figure 1 L T2 cells express AR mRNA. Primers specific for mouse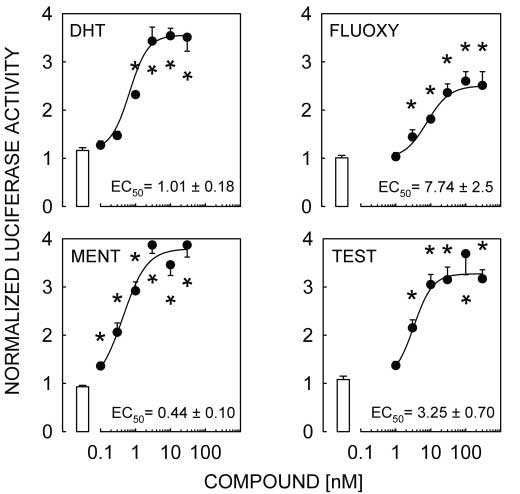 604 M A LAWSON and others · Androgen responsiveness of L T2 cells
Figure 2 DHT, FLUOXY, MENT and TEST activate the pGL3-MMTV reporter transfected
604 M A LAWSON and others · Androgen responsiveness of L T2 cells
Figure 2 DHT, FLUOXY, MENT and TEST activate the pGL3-MMTV reporter transfected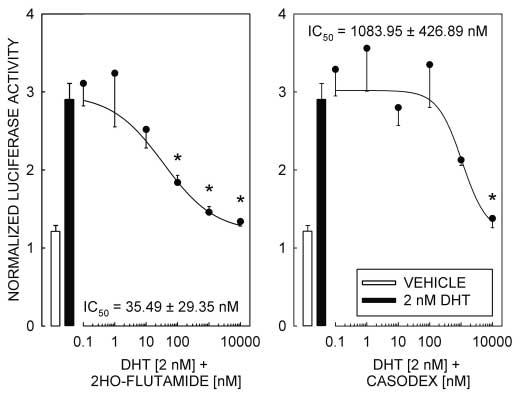 Androgen responsiveness of L T2 cells · M A LAWSON and others 605
Figure 3 AR antagonists 2-hydroxy-flutamide and casodex block DHT-induced reporter
Androgen responsiveness of L T2 cells · M A LAWSON and others 605
Figure 3 AR antagonists 2-hydroxy-flutamide and casodex block DHT-induced reporter